First Patient Begins Newly Approved Sickle Cell Gene Therapy
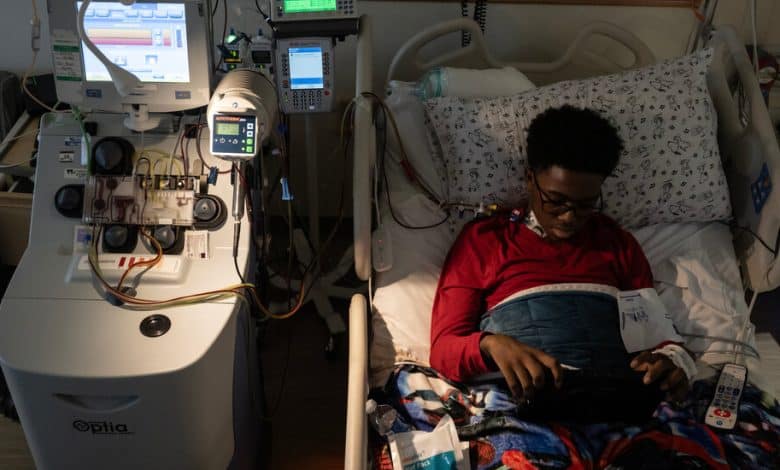
On Wednesday, Kendric Cromer, a 12-year-old boy from a suburb of Washington, became the first person in the world with sickle cell disease to begin a commercially approved gene therapy that may cure the condition.
For the estimated 20,000 people with sickle cell in the United States who qualify for the treatment, the start of Kendric’s monthslong medical journey may offer hope. But it also signals the difficulties patients face as they seek a pair of new sickle cell treatments.
For a lucky few, like Kendric, the treatment could make possible lives they have longed for. A solemn and shy adolescent, he had learned that ordinary activities — riding a bike, going outside on a cold day, playing soccer — could bring on episodes of searing pain.
“Sickle cell always steals my dreams and interrupts all the things I want to do,” he said. Now he feels as if he has a chance for a normal life.
Near the end of last year, the Food and Drug Administration gave two companies authorization to sell gene therapy to people with sickle cell disease — a genetic disorder of red blood cells that causes debilitating pain and other medical problems. An estimated 100,000 people in the United States have sickle cell, most of them Black. People are born with the disease when they inherit the mutated gene for the condition from each parent.
The treatment helped patients in clinical trials, but Kendric is the first commercial patient for Bluebird Bio, a Somerville, Mass., company. Another other company, Vertex Therapeutics of Boston, declined to say if it had started treatment for any patients with its approved CRISPR gene-editing-based remedy.